Attendees List: European Medical Device Manufacturers 2019
$800.00
Attendees List : European Medical Device Manufacturers-2019
Industry: Medical Device Manufacturers
Geography: European
Description
European Medical Device Manufacturers 2019 Email List
- The International Exhibition for European Medical Device Manufacturers- 2019 was held in May at Nuremberg, Germany. The event focused on products and services related to Healthcare, Medical Devices and Technology. Over 6,800 people from across the world, attended this event.
- Our verified European Medical Device Manufacturers 2019 Mailing List helps you to establish contact with your target customers. Also, to achieve high ROI.
Location: Europe
Delivery: Customers can download the list, after the payment is made.
Job titles in our Email List:
- Entrepreneurs
- C-level Executives
- Business Owners
- Healthcare Professionals
Attendees list: Contact addresses of attendees of European Medical Device Manufacturers Exhibition- 2019
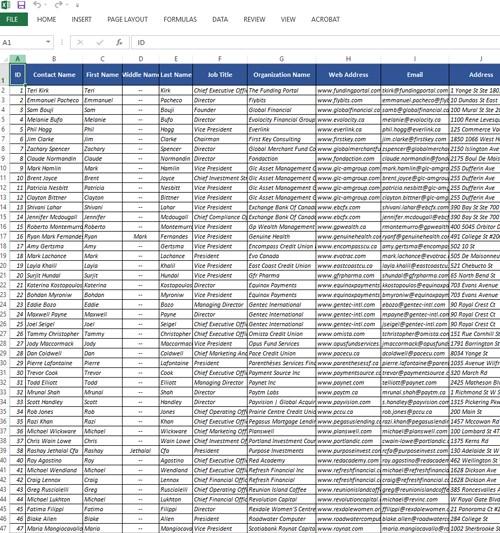
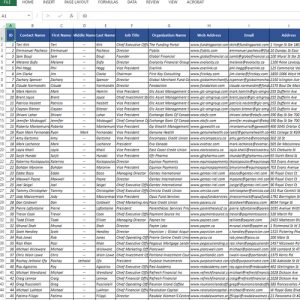
Contacts: Access to over 3,119 permission-based contacts
Data Fields: Our data consists of detailed customer information like:
- Contact names
- Phone numbers
- Mailing addresses
Easy Payment Option: PayPal
Reaching your target customers is no more a tedious task. Avail our European Medical Device Manufacturers 2019 Email List and we will do the rest for you.
So, what are you waiting for? Hurry up! Grab the most updated European Medical Device Manufacturers 2019 Contact Database. Also, receive exciting offers on your first order.
If you have any queries regarding the item(s) purchased, contact us any time and we will get back to you within 12 hours.
About our Attendees lists
Things we check before collecting and building Attendees database
- Recipients in our list have a registered attendance, anticipated attendance (pre-registration) to attend a particular listed show.
- The timeliness of our lists depends upon the schedule of the show. For instance: Current or past edition of the show. Irrespective of the current or past edition of show, we regularly update our master database. Also, we recheck the contacts for accuracy and email deliverability. Hence, it would enhance the credibility of your campaigns.
Steps involved in building our Attendees list
Experts at ProDataLabs conduct regular data quality checks that enhance email deliverability and accuracy. We follow a systematic data building process with high data quality standards. Here are the steps we follow to build a high-quality Attendee Database.
1. We collect customer data from a wide variety of data sources offline and online.
Here are some of those sources.
- Previous year Attendees List
- Online magazines and subscriptions
- Business White Pages Card Collectors
- Business Newswires
- Compilation of specialized trade show Attendance lists
- B2b Portals, and many more.
2. We also buy customer information from vendors who specialize in commercializing their database. These vendors would have already collected the list from trade expos, conferences, or events.
3. After collecting the list, we append the data. We remove the missing or outdated information from the list and fill up the empty fields that lack current customer information.
4. Our telemarketing team verifies the above–prepared information with the prospects. We have included the list of only those prospects, who have agreed to communicate with third parties. Hence, we prepare an opt-in list of attendees by identifying their interest in attending a show, event, expo, or conference.
5. Our company or compilers do not hold any relationship with the listed event. We compile and collate this information for each event with the above collection efforts. Also, we don’t market the lists as an official buyer list.
Customized samples available on special request Contact Us
2 reviews for Attendees List: European Medical Device Manufacturers 2019
Only logged in customers who have purchased this product may leave a review.







Zavier Bailey (store manager) –
We had purchased this list from them about a month ago and there were a little up’s and down’s but we were able to achieve our targets and at the end that’s all that matters.
Archie Norman –
The European Medical Device Manufacturers list got us connected too many C- level executives. I am happy with my decision and will definitely continue a long term business relationship with them.